

What is the mechanism by which the principle operates, and where does it apply? If we take a look at atoms, we can see that once they obtain a new electron or electrons, they usually migrate to the lowest energy state or shift to the outermost shell of the atom.

Applicationsįor most applications in chemistry, the rule is used to explain or identify the electron shell structure of atoms and to anticipate which atoms are most likely to contribute electrons. Albert Einstein himself recommended him for the honour, which he received. The fermions described by the principle of conservation of momentum comprise elementary particles like quarks, electrons, neutrinos, and baryons, among others.Īs well as being given the Nobel Prize in Physics, Wolfgang Pauli received the award in 1945 for his discovery of the Pauli Exclusion principle as well as his overall contribution to the field of quantum mechanics. Later in the year 1940, he extended the principle to include all fermions under the terms of his spin-statistics theorem, which was published the following year. He was able to describe the behaviour of electrons in its simplest form using this approach. In the year 1925, an Austrian scientist by the name of Wolfgang Pauli proposed the principle. Formalisation of the Fundamental Principle

The Bose-Einstein distribution function, on the other hand, is the source of the term “boson” itself. In terms of nomenclature, fermions are named after the Fermi–Dirac statistical distribution that they follow, which is derived from quantum mechanics. Furthermore, unlike fermions, bosons are capable of sharing or having the same quantum states. The fact that particles having an integer spin, such as bosons, have symmetric wave functions does not matter in this case. It applies to fermions and other particles with half-integer spin, as well as to bosons. Pauli’s Exclusion Principle, on the other hand, does not simply apply to electrons. The two electrons that are present in the same orbital must have opposite spins or they must be antiparallel in order for them to be in the same orbital.Only two electrons can share the same orbital at the same time.According to the Pauli Exclusion Principle, there are two important rules that must be followed: To put it another way, every electron should have or be in a state that is distinct from the others (singlet state). The Pauli exclusion principle asserts that no two electrons in a single atom will have an identical set of quantum numbers or will have the same quantum state (n, l, ml, and ms). What Is The Pauli Exclusion Principle, And How Does It Work As a result of this section, we will gain a thorough understanding of the Pauli exclusion principle and all of its underlying principles. As a general rule, Pauli’s exclusion principle aids in our understanding of electron configurations in atoms and molecules, as well as providing an explanation for the periodic table’s classification of elements.
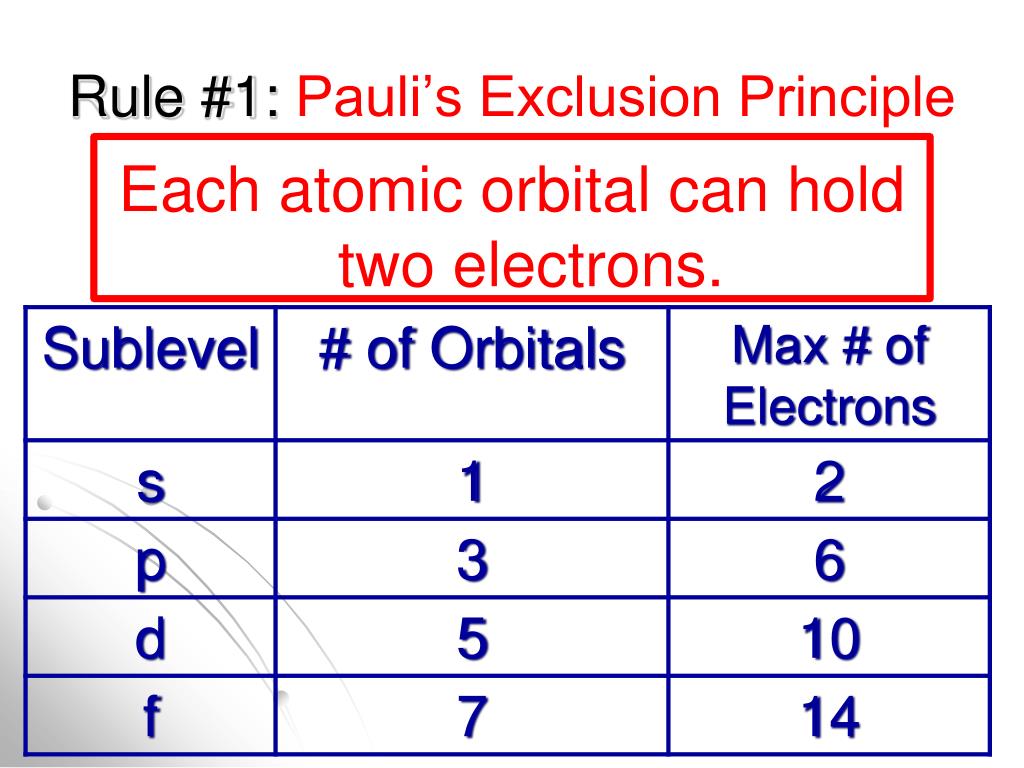
Having a basic understanding of it is essential for pupils, especially when they are studying electrons.


 0 kommentar(er)
0 kommentar(er)
